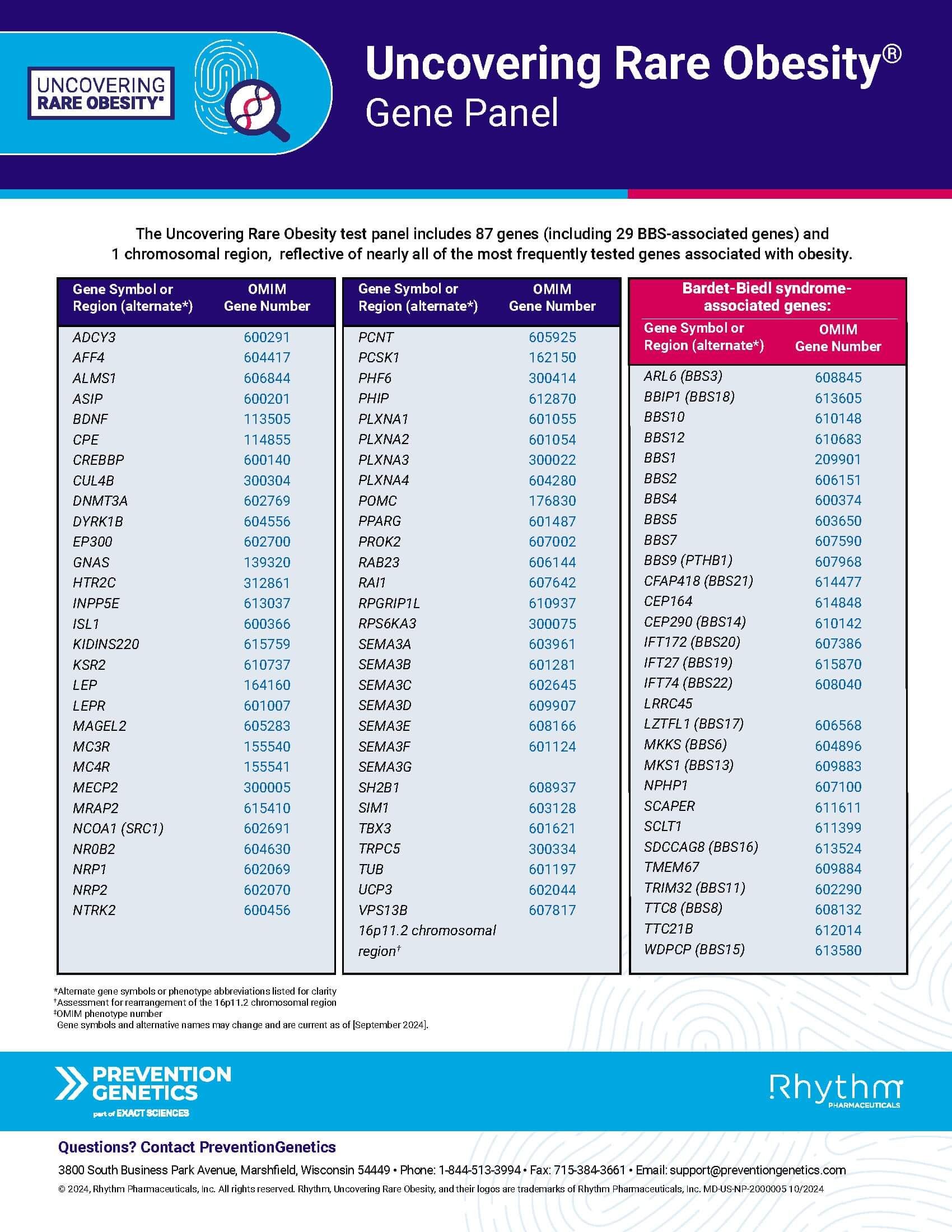
Monogenic Obesity
Rare monogenic diseases that cause obesity
Actor portrayals
Rare monogenic diseases that cause obesity are likely underdiagnosed
These diseases are characterized by 2 key features related to weight: hyperphagia and early-onset obesity.1
People affected by rare monogenic obesity may present with additional clinical characteristics, including neurological, growth, and endocrine abnormalities, as well as a family history of weight differences between family members.2,3
What are the unique signs and symptoms associated with monogenic obesity?
POMC deficiency1–5
Signs and symptoms of
proopiomelanocortin (POMC) deficiency
may include:
- Early-onset, severe obesity
- Hyperphagia
- Endocrine abnormalities
PCSK1 deficiency1–4,6–8
Signs and symptoms of proprotein convertase subtilisin/kexin type 1 (PCSK1) deficiency may include:
- Early-onset, severe obesity
- Hyperphagia
- Postnatal diarrhea within the first weeks of life
- Hyperinsulinemia
- Hypoglycemia
- Metabolic acidosis
LEPR deficiency1,2,9
Signs and symptoms of leptin receptor (LEPR) deficiency may include:
- Early-onset, severe obesity
- Hyperphagia
- Hypogonadotropic hypogonadism
Additional diseases2,3,10,11
There are other diseases with early-onset obesity and hyperphagia due to melanocortin-4 receptor (MC4R) pathway impairment.
These rare monogenic diseases also have unique signs and symptoms:
- SH2B1 deficiency: Insulin resistance
- SRC1 deficiency: Hyperleptinemia and insulin resistance
Genetic testing through the Uncovering Rare Obesity® program is available for eligible patients. For more information about the genetic testing program, visit
Downloadable
Genetic testing may help aid in diagnosis. Recognize the genes most frequently associated with genetic diseases that cause obesity.
Patterns of inheritance
Rare genetic diseases that cause obesity are inherited in several patterns, depending on the gene involved and whether the related genotype is dominant or recessive.
In autosomal recessive inheritance12
- 2 copies of variant allele (biallelic genotypes) are required for the phenotype to be present (e.g., homozygous or compound heterozygous variants are required)
- Both parents of the affected individual are likely carriers of a single variant
- Not typically observed in every generation
Biallelic Genotypes
Homozygous
Compound
Heterozygous
In autosomal dominant inheritance13
- 1 copy of the allele is sufficient for the phenotype to be present (e.g., heterozygous variants are sufficient)
- The affected individual likely has 1 affected parent
- Disease can occur in every generation
Heterozygous Genotypes
Heterozygous
Multiple
Heterozygous
(same allele)
Genetic testing may help reveal disease-causing genetic variants. The American College of Medical Genetics and Genomics (ACMG) classifies variants into 5 categories.14
Pathogenic
Likely Pathogenic
Variant of Uncertain Significance (VUS)
Likely Benign*
Benign*
Understanding the role of the hypothalamic MC4R pathway in obesity and hunger
The MC4R pathway regulates hunger, satiety, and energy balance.1,15,16
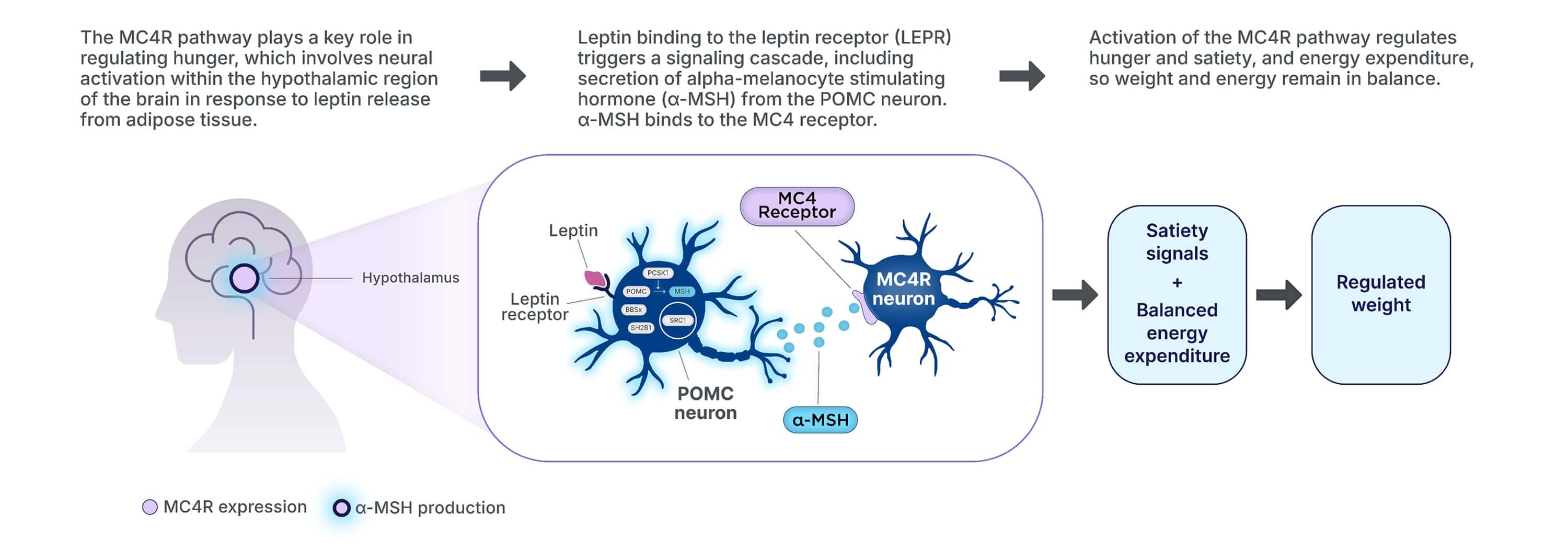
Genetic dysfunction in the MC4R pathway can impair MC4R pathway signaling,
ultimately leading to accelerated and sustained weight gain.1,2,16
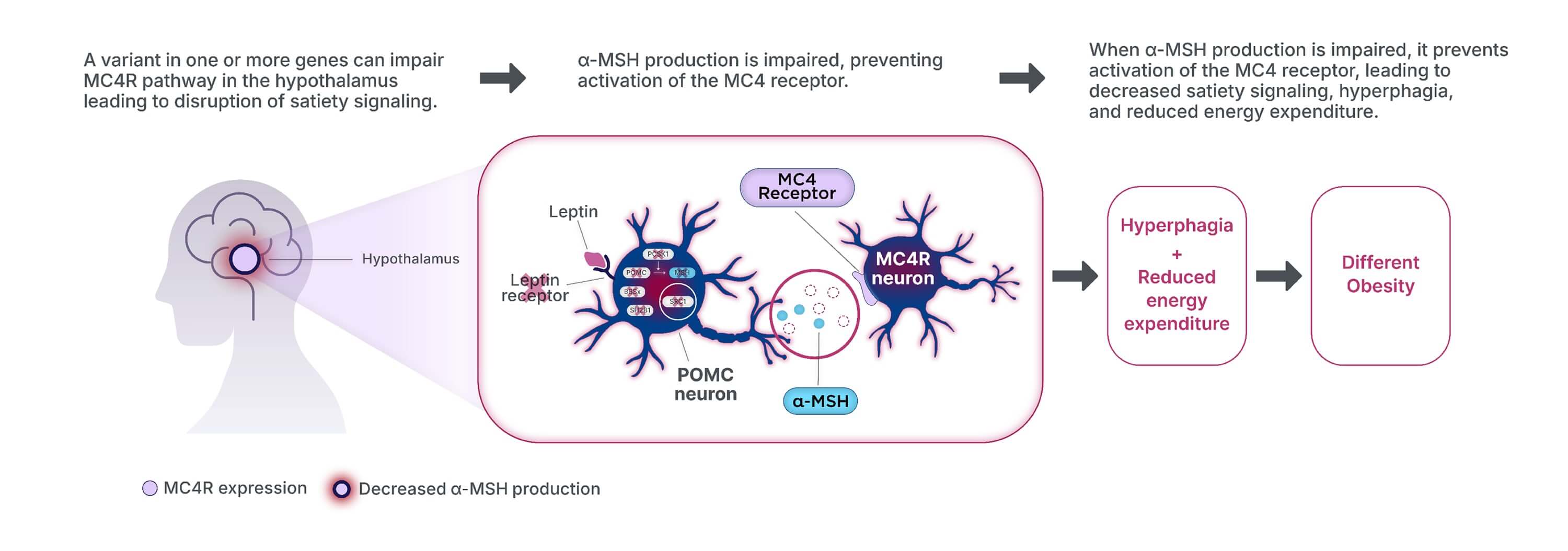
POMC=Proopiomelanocortin