Mechanism of Disease
Different obesity defined
Actor portrayal
Understanding the role of the hypothalamic
MC4R pathway
The melanocortin-4 receptor (MC4R) pathway in the hypothalamus plays a critical role in energy balance and body weight regulation. The key distinguishing symptoms of MC4R pathway impairment include hyperphagia and obesity.1,2
General obesity and MC4R pathway–driven obesity are not the same
General Obesity3,4
Occurrence: Can occur at any age, including later in life.
Cause: Interaction of multiple factors, including:
- Age, race, or gender
- Concurrent illnesses
- Common genetic variants
- Concomitant medications
- Environmental factors
- Nutrition and physical activity
MC4R Pathway–Driven Obesity1,2,5–8
Occurrence:
- With genetic diseases, MC4R pathway impairment is present at birth, leading to early-onset obesity
- With acquired forms, impairment of the pathway will follow injury or trauma to the hypothalamus, leading to weight gain
Cause: Impairment of the MC4R pathway, leading to:
- Hyperphagia (chronic pathological condition characterized by insatiable hunger, impaired satiety, and persistent abnormal food-seeking behaviors)
- Obesity
Hyperphagia severity can vary
Hyperphagia is often differentiated from other types of overeating by its severity and persistence. However, the presentation of symptoms and behaviors can vary in severity from patient to patient and based on the underlying disease.2,9
Downloadable
Recognize different obesity. Take action and screen your patients. Identify hyperphagia in your patients who may have MC4R pathway impairment.
Causes of MC4R pathway impairment
Knowing the cause of your patient's different obesity and hunger may
help manage long-term health outcomes and alleviate patient burden.1,2,10
Acquired5-8
- Injury to the hypothalamus can impair MC4R pathway signaling, ultimately leading to weight gain
Genetic1,2
- Some gene variants can result in a rare genetic disease and lead to impairment of MC4R pathway signaling. While symptoms can vary depending on whether it is syndromic or monogenic, MC4R pathway impairment due to genetic variants can lead to hyperphagia and early-onset obesity

Why having a diagnosis matters
Obesity due to MC4R pathway impairment requires a distinct diagnosis and tailored management compared with the management of general obesity.
Role of the hypothalamus and MC4R pathway
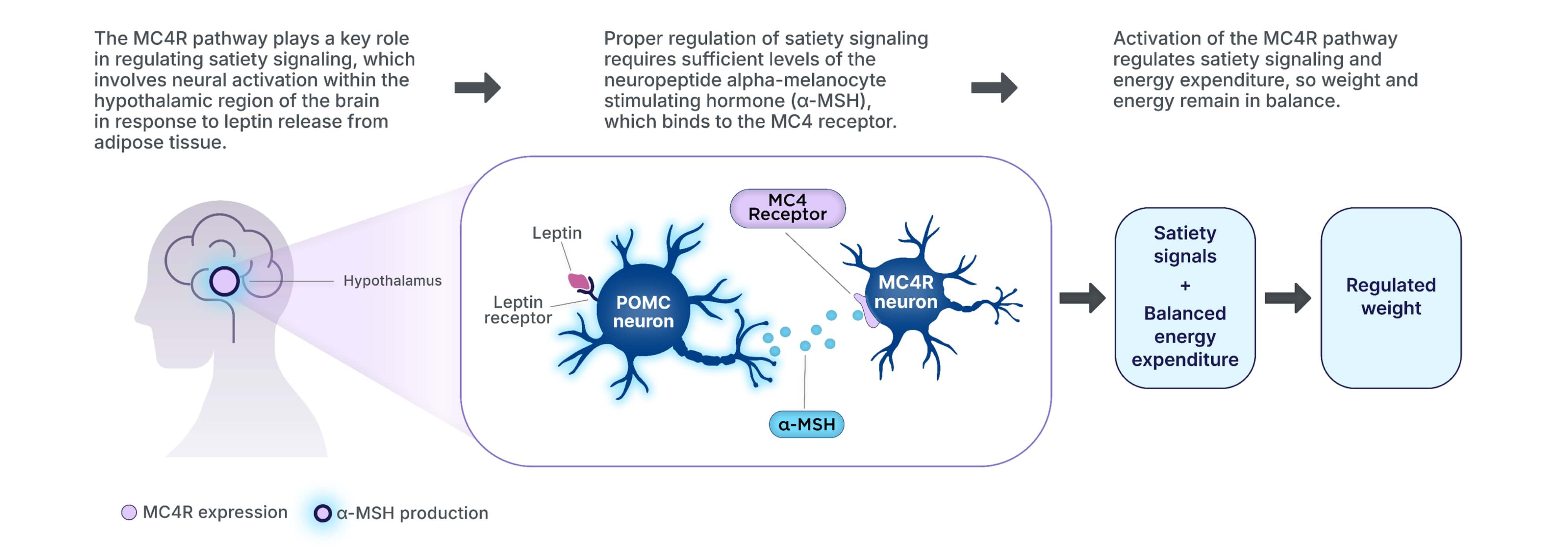
The MC4R pathway in the hypothalamus is a key signaling pathway in regulating
hunger, satiety, energy expenditure, and ultimately, weight3,11–14
Impairment in the MC4R pathway
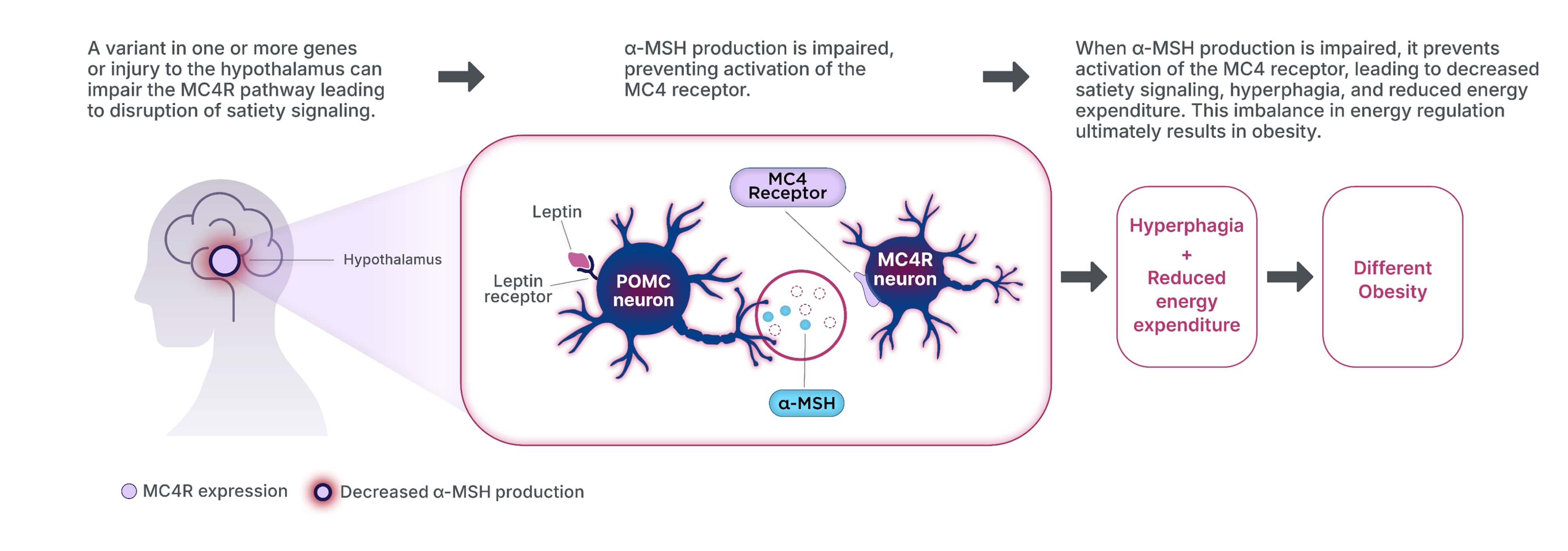
MC4R pathway impairment disrupts MC4R pathway signaling, ultimately leading to
hyperphagia and decreased energy expenditure, resulting in weight gain1,3,11–16
POMC=Proopiomelanocortin
