Acquired hypothalamic obesity
Acquired HO
Actor portrayal
Acquired hypothalamic obesity (HO) is characterized by hypothalamic injury leading to weight gain1-4
Healthy Hypothalamus5,6
The hypothalamus plays a key role in many diverse functions, including regulating:
Satiety and hunger
Energy expenditure
Body weight
Patients with hypothalamic dysfunction due to injury experience a variety of health concerns, one of which is acquired HO.5,6
Acquired Hypothalamic Obesity5,6
Unlike general obesity, acquired HO is characterized by distinct factors that can contribute to weight gain:
Hyperphagia
Decreased energy expenditure
Weight gain
Differences in type, location, and extent of hypothalamic injury may result in variable time to onset and progression of weight gain.5,7
While HO can be congenital or acquired, more than 80% of cases are acquired.8
Learn more about energy expenditure in acquired HO.
Questions about acquired hypothalamic obesity?
Causes of acquired HO
Known Common Causes8:
- Hypothalamic-pituitary tumors, including craniopharyngioma, astrocytoma, and pituitary adenomas
- Tumor treatment, including surgical resection and radiotherapy
Other Causes8:
- Traumatic brain injury
- Hypothalamic inflammation
- Stroke
Know who is at risk—acquired HO occurs in up to 75% of patients with craniopharyngioma following treatment.9
Mechanism of disease
Acquired HO has an underlying pathophysiology that distinguishes it from general obesity.1,2,4
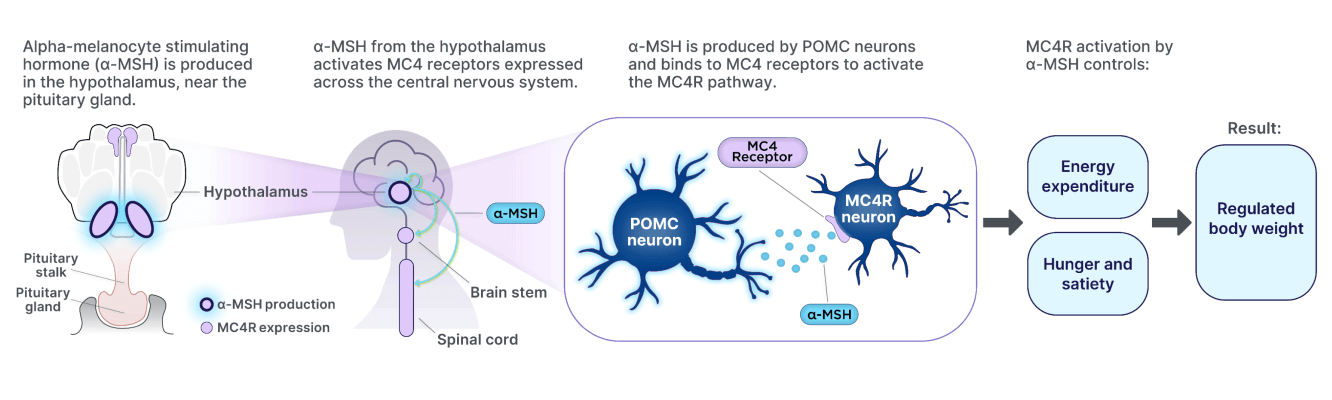
The melanocortin-4 receptor (MC4R) pathway regulates energy balance and weight.10–12
Injury to the hypothalamus can disrupt MC4R pathway signaling, ultimately leading to weight gain.1,2,4
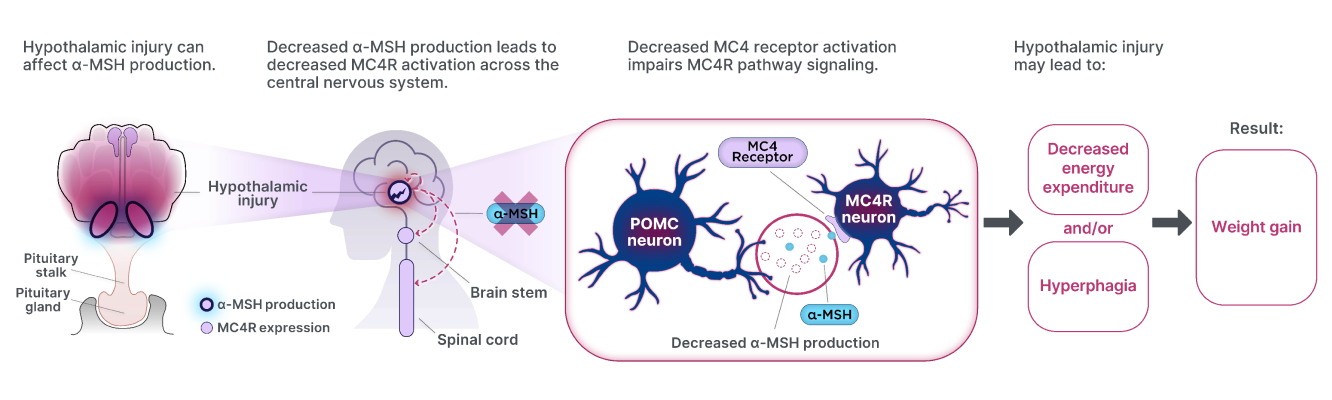
POMC=Proopiomelanocortin